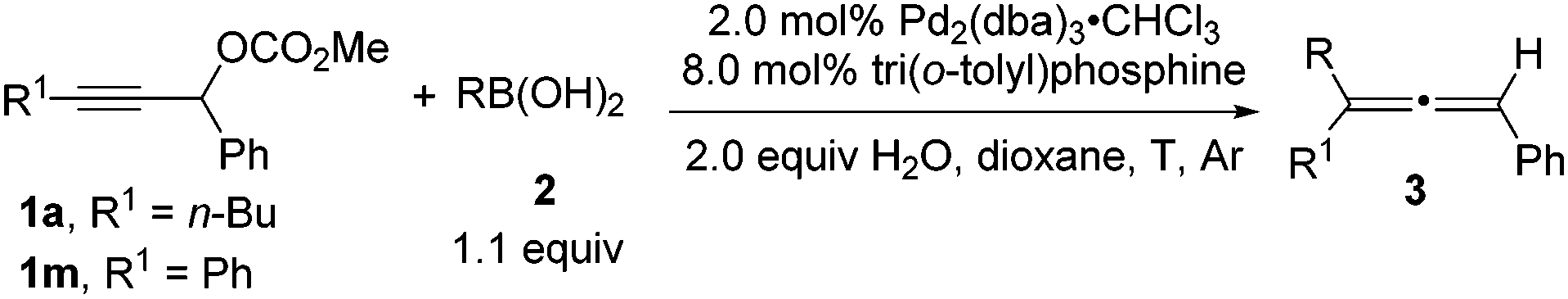Convenient and General Palladium‐Catalyzed Carbonylative Sonogashira Coupling of Aryl Amines - Wu - 2011 - Angewandte Chemie International Edition - Wiley Online Library

China Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) CAS No.: 14221-01-3 Manufacturers - Free Sample - Alfa Chemical

Cationic palladium(II)–acetylacetonate complexes containing phosphine and aminophosphine ligands and their catalytic activities in telomerization of 1,3-butadiene with methanol - ScienceDirect

CHAPTER 1 New Chemistry for Organic Photovoltaic Materials (RSC Publishing) DOI:10.1039/9781782622307-00001
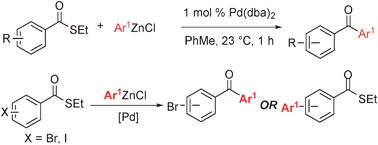
Synthesis of diaryl ketonesvia a phosphine-free Fukuyama reaction - Chemical Communications (RSC Publishing)
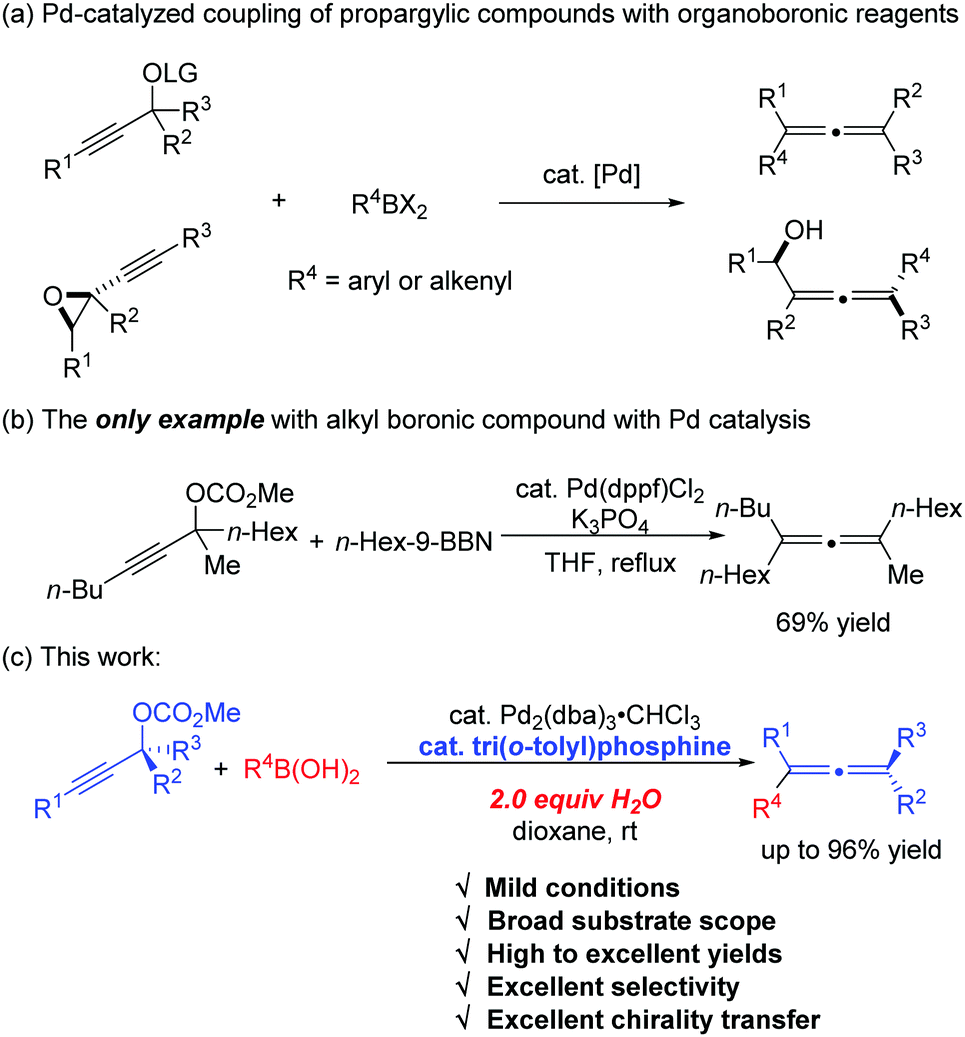
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)

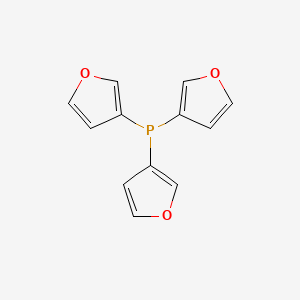
Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect

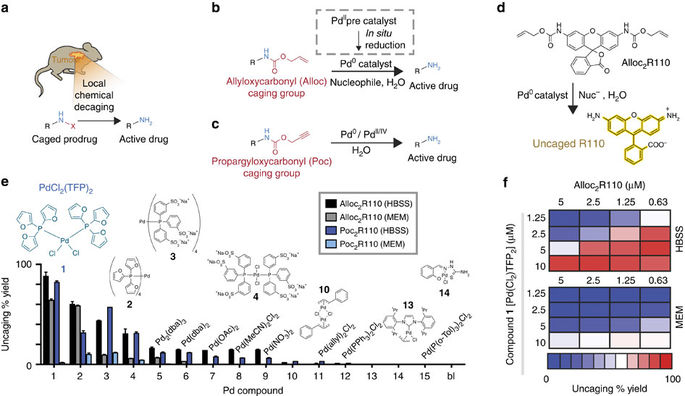
Activation of AllylUE (1) and PropUE (2) probes in presence of Pd or Pt... | Download Scientific Diagram

Resorcinarene‐Based o‐Biarylphosphines in Palladium‐Catalysed Suzuki–Miyaura Cross‐Coupling Reactions of Bulky Substrates - Elaieb - 2017 - European Journal of Inorganic Chemistry - Wiley Online Library